

Barium is used as paper coating pigment.
Used in paints and varnishes as a filler.Barium is used in making drilling mud, that is efficiently used in drilling through rocks.It is used in making fluorescent lamps.It is widely used in making barium-nickel alloys in spark-plug electrodes.Barium sulfate is important in petroleum industry as drilling fluids in oil and gas wells.
#BA ATOMIC NUMBER TV#
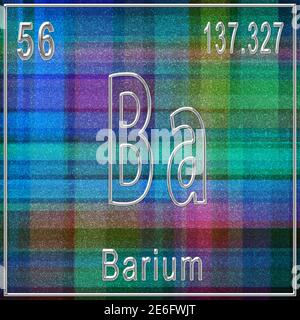
Used to remove unwanted gases from vacuum tubes such as TV pictures tubes.Volatile barium compound burns with a green to pale green flame. Barium hydroxide unlike calcium hydroxide absorbs little CO 2 in aqueous solution insensitive to atmospheric fluctuation. They are denser than strontium or calcium analogs except for halides. Compoundsīarium salts are white when solids and colorless when dissolved. When reacted with non-metals barium form poisonous compounds. Barium combines with metals such as aluminum, zinc, lead and zinc forming alloys. Barium reacts with ammonia form complexes such as Ba (NH 3) 6. In alkaline family radium is more reactive. It is so reactive that it must be stored under kerosene, petroleum, or some other oily liquid to prevent it from reacting with oxygen and moisture in the air. It combines easily with oxygen, the halogens, and other non-metals also reacts with water and most acids. Reaction with chalcogens are highly exothermic. Chemical propertiesīarium chemically resembles, magnesium calcium and strontium but is more reactive. The atomic number of barium is 56 and atomic weight of 137.327 and belong to Group 2 periodic table. Barium has specific weight and good electrical conductivity. When heated, barium compounds give off a pale yellow-green flame. Its density is 3.6 grams per cubic centimeter. It has a melting point of about 725☌ and a boiling point of about 1,640☌. It is comparatively dense and reactive alkaline earth metal. Pure barium is a silvery-white shiny malleable metal. The major ore of barium that are widely used for the extraction of barium is barite. Such activities include mining, oil combustion and coal combustion. Various anthropogenic activities have led to increased concentration of barium in the environment, including soil, water and air. The barium mineral benitoite, occurs as a very rare blue fluorescent gemstone, and is considered as the official gem stone of California.

High amounts of barium are also found in soils and in plants, fish, seaweeds, and in nuts . The world’s major sources of barium ores are UK, China, Czech Republic, India, Morocco, the United States, Turkey, and Italy. Witherite ore containing barium carbonate BaCO 3. The most common source of barium is barite and witherite. in nature, barium mostly occurs in combination with sulfur, oxygen and carbon. Barium Periodic Table Classificationīarium is 14 th most abundant element in earth crust. The name barium have been derived from the Greek word barys that mean heavy. Barium was identified as a new element in 1774 but not isolated in its pure form via electrolysis in 1808. In 1600, Vincenzo Casciarolo found unusual pebbles that were when exposed to heat would shine during night, named barite (Barium Sulfate BaSO 4).
#BA ATOMIC NUMBER FREE#
Because of its high chemical reactivity, barium is never found in nature as a free element Discovery and History It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. To get just the number of neutrons you take the difference between protons and mass number, thus #137-56=81#.Barium is a chemical element with symbol Ba and atomic number 56.
#BA ATOMIC NUMBER PLUS#
And the #137# for the mass number designates #137# total protons protons plus neutrons. Thus the "barium" in "barium 137" means #56# protons. When the element is barium, that means the aomic number is #56# which you see by looking for barium on a periodic table (or nowdays by entering "barium" into an online search engine). That's why chemists commonly identify an isotope with the element, which implies its atomic number, and then the mass number. Mass number is also a key property of atoms because we need to know the masses of atoms to understand how a given amount of material we weigh out will react - for instance, how much carbon dioxide gas can we get out of 10 grams of calcium carbonate and how much hydrochloric acid do we need to get all the gas? We can write a balanced equation #"CaCO"_3+2 "HCl" rarr "CaCl"_2+"H"_2"O"+"CO"_2#, but to match that with what we weigh we need to know atomic masses. This is roughly proportional to the total mass of the atom. Mass number is the number of protons plus neutrons. The atomic number determines what an element is chemically, for the number of protons determines the number of electrons needed to balance the charges in an atom, which in turn determines the electron configuration the atom will adopt. Two numbers are used to identify a nucleus:Ītomic number - number of just protons in the nucleus.


 0 kommentar(er)
0 kommentar(er)
